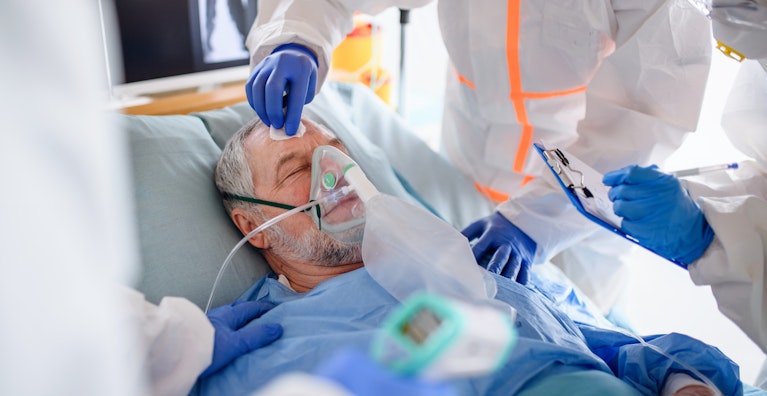
Latest Management Strategies for Hospitalized COVID-19 Patients
Author: Dr. Guneet J Mann, MD
The disease spectrum of COVID-19 is very wide. On the one hand are the asymptomatic or mildly symptomatic patients and on the other hand are patients who are fighting for their lives in the intensive care units. A significant number of hospitalized patients undergo severe inflammatory reactions triggered by SARS-CoV-2. Besides supportive therapy, more aggressive treatment strategies are being increasingly used with variable degrees of success.
Blood Purifying Devices
Extracorporeal blood purification (EBP) is a treatment in which blood from a patient is passed through a device (e.g. a membrane or a sorbent) in which a solute (waste products, toxins) and possibly water are also removed. When fluid is also removed, replacement fluid is added. EBP is used primarily in patients with renal failure (Renal Replacement Therapy-RRT). More than twenty years ago, it was seen that RRT could also remove inflammatory mediators from the plasma of septic patients. A survival benefit with hemofiltration was also noted later. Hence, started the use of blood purification as a treatment for human septic shock.
The role of cytokine storm and hyper inflammation, on the severity of COVID-19 patients has been well corroborated in different studies. In an article published in Intensive Care, Ruan et al documented high concentrations of inflammatory biomarkers like C- reactive protein, ferritin and IL-6 in COVID-19 patients who died. They concluded that COVID-19 mortality may be due to virus activated “Cytokine Storm Syndrome” or fulminant myocarditis.
Blood purification devices have now been given permission for use in severe cases of COVID-19 in a number of countries. One brand of blood purifying devices, CytoSorb, was used to treat more than seventy critically ill COVID-19 patients and specifically added to Coronavirus treatment guidelines in Italy and Panama, March 25, 2020. The use of CytoSorb has now begun in seriously ill COVID-19 patients who have cytokine storm and life threatening complications such as Acute Respiratory Distress Syndrome (ARDS), in Italy, China, Germany and France. Preliminary data of the seventy patients tested, shows that there is a marked reduction in Cytokine storm and inflammation, improved lung functions, weaning from mechanical ventilation and a reversal of shock.
CytoSorb is now specifically recommended in the Italy Brescia Renal COVID Task Force Guidelines for treating patients with severe COVID-19 infection and stage 3 renal failure on continuous RRT, published in Italian Society of Nephrology website. Blood purification in COVID-19 infections is also recommended to treat Cytokine Storm by the National Health Commission in China. This technology has been used in thousands of Extra Corporeal Membrane Oxygenation (ECMO) treatments to date, in non COVID-19 patients around the world.
On April 10, 2020, US FDA gave emergency use authorization (EUA) for a pair of Blood Purification Systems to treat adult COVID-19 patients admitted in ICU. The EUA applies to Trumo BCT Inc’s Spectra Optia Apheresis and Marker Therapeutics AG’s Depuro D2000 Adsorption Cartridge. On April 13, 2020 it gave EUA for the CytoSorbent System too.
Mesenchymal Stem Cell Transplantation (MSC)
Umbilical Cord derived Mesenchymal Stem Cells (MSCs) have been used safely and effectively for immune mediated inflammatory diseases such as Graft Versus Host Disease (GVHD) and Systemic Lupus Erythematosus (SLE). MSCs play a positive role in two ways, by immunomodulation and by their ability to differentiate. Immunomodulatory actions are related to secretion of cytokines and because of the direct interactions with immune cells. The immunomodulatory effects are further enhanced by the activation of Toll-Like Receptors (TLR) in MSCs by pathogen associated molecules such as Lipopolysaccharides (LPS) or single stranded RNA from viruses like SARS-CoV-2.
The first step in the pathogenesis of COVID-19 is the entry of SARS-CoV-2 into human cells by attaching to Angiotensin Converting Enzyme-2 (ACE-2) receptors by its spike proteins. A research team from Germany revealed that cellular serine protease TMPRSS2, for SARS-CoV-2 Spike Protein priming, is also important for host cell entry and spread.
ACE2 receptors are present widely on tissues in the body, like type 2 Pneumocytes of lungs, kidney, intestines, capillary endothelium etc. However, the immune cells such as T and B lymphocytes and macrophages, in the bone marrow, lymph nodes, thymus, and the spleen, are all negative for ACE-2 receptors. MSCs are also ACE-2 receptor and TMPRSS2 negative. This makes them immune to SARS-CoV-2. This is the basis of using them in severe COVID-19 infections.
The viral infection causes a total failure of function of lymphocytes and almost the whole immune system. Several studies have reported lymphopenia and high levels of C-reactive protein in COVID-19 patients with severe infections. MSCs play a role by reversing the lymphocyte subsets, mainly through dendritic cells. The interactions of MSCs with the dendritic cells leads to a shift of the immune system from T helper 1 to T helper 2 type of responses.
After entering the human body through the intravenous route, part of the MSCs accumulate in the lung. There, they probably improve the microenvironment, protect alveolar epithelial cells, prevents pulmonary fibrosis and improve lung functions. Due to their immunosuppressive capacity, MSCs significantly decrease the serum levels of pro inflammatory cytokines and chemokines. This leads to decreased attraction of mononuclear/macrophages to the fragile lung, at the same time recruiting more regulatory dendritic cells to the sites of inflammation. They also increase IL-10 and Vascular Endothelial Growth Factor (VEGF), which promotes lung repair.
US FDA has authorized Umbilical cord derived Mesenchymal Stem Cell (MSC) transplant, to prevent life threatening lung inflammation that accompanies severe cases of COVID-19. They have provided authorization for a twenty four patient clinical trial for such patients.
An article related to the use of MSCs in COVID-19 pneumonia, was published by Leng Zikuan, Zhu Ronjia, Hou Wei, et al in the Journal of Aging and Disease, February28, 2020. They studied the effects of MSC transplant on seven COVID-19 patients with pneumonia. A favorable result was reported in all of them.
Use of Convalescent Sera in COVID-19 Patients
Immunity is of two types- active and passive. Active immunity is when the human body mounts an immune reaction in response to an invading microorganism. Passive immunity is when the body is not actively involved in producing immunity. This involves introducing preformed antibodies into the human body through various routes. A classic example of this is the newborn receiving maternal antibodies that protect the newborn till the age of around six months.
Convalescent Sera has been used as early as the twentieth century, to stem the outbreak of viral diseases such as poliomyelitis, measles, mumps and influenza. In the 2009-2010 H1N1 influenza virus pandemic, convalescent sera was used to treat patients with severe H1N1 disease, requiring intensive care. Convalescent serum was also used in the 2013 West African Ebola Virus epidemic. In previous epidemics of Coronaviruses, SARS 1 in 2003 and MERS in 2012, Convalescent Sera was used for patients who were hospitalized.
Recently on April 13, 2020, US FDA issued guidelines to health care providers and investigators on the administration and study of convalescent plasma, collected from individuals who have recovered from COVID-19. COVID-19 convalescent plasma has not yet been approved for use by FDA. It is regulated as an investigational product. Eligibility criteria for the potential patients:
- Laboratory confirmed COVID-19.
- Severe or immediately life threatening COVID-19, for example:
- Severe disease is defined as one of the following:
- Shortness of breath (dyspnea)
- Respiratory frequency ≥ 30
- Blood oxygen saturation ≤ 93%
- Ratio of partial pressure of arterial oxygen to fraction of inspired oxygen < 300.
- Lung infiltrates > 50% within 24-48 hours.
- Life threatening disease is defined as one or more of the following:
- Respiratory failure.
- Septic shock
- Multiple organ dysfunction or failure.
- Severe disease is defined as one of the following:
- Informed consent provided by the patient or a health care proxy.
So, the research continues, as the pandemic spreads. Scientists, epidemiologists and researchers are working at a fast and furious pace along with the governments of their countries, to expand their knowledge of the disease, its diagnosis and management.
References:
- Houschyar KS, Pyles MN, Rein S, Neitzschmann I, Ducher D, Maan ZN, Weissenberg K, Philipps HM, Strauss C, Reichelt, Siemers F. Continuous hemadsorption with a cytokine adsorber during sepsis - a review of literature. International Journal of Artificial Organs.2017 May 29; 40(5)205-211.
- Park WH. Therapeutic use of antipoliomyelitits serum in preparalytic cases of poliomyelitis. JAMA. 1932; 99:1050–1053.
- Park WH, Freeman RG. The prophylactic use of measles convalescent serum. JAMA. 1926; 87(8):556–558.
- Gallagher JR. Use of convalescent measles serum to control measles in a preparatory school. Am J Public Health Nations Health. 1935; 25(5):595–598.
- Rambar AC. Mumps; use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child. 1946; 71:1–13.
- Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010; 38(4 suppl):e66–e73.
Source:
Share this on Social media
HEALTH DISCLAIMER
This blog provides general information and discussions about health and related subjects. The information and other content provided in this blog, or in any linked materials, are not intended and should not be construed as medical advice, nor is the information a substitute for professional medical expertise or treatment.
The content is for information purpose only and is not a medical advice. Qualified doctors have gathered information from reputable sources; however Credence Medicure Corporation is not responsible for errors or omissions in reporting or explanations. No individual should use the information, resources and tools contained herein to self diagnose or self treat any medical condition.
If you or any other person has a medical concern, you should consult with your health care provider or seek other professional medical treatment. Never disregard professional medical advice or delay in seeking it because of something that have read on this blog or in any linked materials. If you think you may have a medical emergency, call your doctor or emergency services immediately.
The opinions and views expressed on this blog and website have no relation to those of any academic, hospital, health practice or other institution.
Credence Medicure Corporation gives no assurance or warranty regarding the accuracy, timeliness or applicability of the content.
comments powered by Disqus